By: Yoko Miura, MD
Disease Background
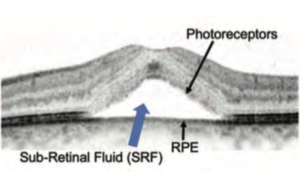
Central serous chorioretinopathy (CSC) is one of the major diseases of the central macula. It occurs mainly in adults between 20 to 50 years of age, and is more prevalent in males; however, the number of female patients has been increasing more over recent years. In this macular disease, the fluid accumulates in the sub-retinal space between the photoreceptor outer segments and the retinal pigment epithelium (RPE).This causes serous retinal detachment (SRD) (Figure 1). Each patient may experience different symptoms, and these could include: blurred vision, distorted vision, hyperopic shift, micropsia, disturbance of color, and darker or lighter vision. Causes and risk factors of CSC include stress, type-A personality, hypertension, exogenous glucocorticoid, hyperopic eye (short axial length), kidney disease, sleep disturbance, smoking, depression (or depression medication), pregnancy, and genetic factors.
Diagnosis and Treatment Approach Planning
When diagnosing CSC, it is very important to use different diagnostic techniques in order to determine the appropriate treatment approach. One of the most useful tools is optical coherence tomography (OCT), which shows SRD with the accumulation of sub-retinal fluid (SRF), presented typically as a homogeneous hypo-reflective space. In OCT, using an enhanced depth imaging (EDI) is recommended because it could indicate a pachychoroid (thickened choroid), to which in some CSC cases are strongly related. Fluorescein angiography (FA) is also very important because it is the only method to see the point(s) of dye leakage from the disrupted RPE barrier function (local or diffuse, single or multiple). This result may directly indicate where the sub-retinal fluid is coming from. Other diagnostic approaches include indocyanine green angiography (ICGA), which is useful to detect choroidal neovascularization (CNV) and abnormal choroidal circulation. Optical coherence tomography angiography (OCTA) is another approach to check CNV, where the retinal and choroidal vessels can be visualized, without injections (unlike with FA
where injections are needed). This alternative approach is useful for patients who may be allergic to fluorescein; and thus, FA cannot be conducted. In case of existence of the active (leaky) CNV, the treatment with anti-vascular endothelial growth factor (VEGF) is at least required.
CSC Cause and Prognosis
Acute CSC is often self-limiting and shows spontaneous resolution within 3 to 4 months. Therefore, in many cases, spontaneous resolution occurs without any active intervention. However, recurrence of acute CSC may reportedly occur within 12 months in 30-50% of cases [1]. Repeated recurrences of CSC may cause degenerative changes in the RPE and photoreceptors, resulting in diffuse leakage and decreased neural function, typically seen in the form of chronic CSC. Chronic CSC occurs in about 5% of total CSC cases, but there are more patients who suffer from repeated CSC and experience worsened visual acuity as a result. Furthermore, as patients get older, especially after multiple recurrences of CSC, it becomes more difficult to recover from SRD, and this may result in poor visual prognosis. In conclusion, early treatment is important in order to reduce the duration of SRD and avoid degeneration of the RPE, and neural retina.
Treatment of CSC
In the past, conventional photocoagulation was applied at the leakage point using an argon laser or dye laser; however, if the leakage point was located close to the fovea, no laser treatment was conducted. The white spots that are ophthalmoscopically seen after photocoagulation indicate destruction of the photoreceptor, and eventually the inner retinal layer. This may result in scar formation, and if it occurs in the central macula, it leaves a permanent dark area in the central visual field, known as scotoma. Today there are some other approaches which can be introduced to avoid this problem, including anti-VEGF intravitreal injections, oral administration of medication such as Eplerenon or Acetazolamide, topical administration (eye drops) of non-steroid anti-inflammatory drugs (NSAID), photodynamic therapy (PDT), and sub-threshold microsecond laser technology including LIGHTMED’s proprietary SP-Mode®.
Retina Laser Therapy with SP-Mode® Microsecond Laser Technology
SP-Mode® enables the retina laser therapy with sub-visible laser irradiation using microsecond pulsing. This technology is available in LIGHTMED’s TruScan and LIGHTLas products. In the continuous wave (CW) mode, used in conventional photocoagulation, the laser pulse is consistently “on” during the whole irradiation time (for example 200ms). On the other hand, with SP-Mode® Microsecond Laser Technology, the laser can repeat on/off times which results in pulsing during irradiation. Typically the repetition rate is 500Hz, which means that the on/off cycle is repeated every 2ms (2000μs). Proportion of the duration of “on” time in an on/off cycle is called the duty cycle (%), equal to (on-time) / (on+off time). For example, a 10% duty cycle would be 10% (200μs) on-time + 90% (1800μs) off-time within one cycle. This is repeated during the determined irradiation time period (e.g. 200ms). During SP-Mode®, the increased temperature decreases during the off times to some extent. Therefore, the total temperature increase is much lower compared to CW mode at the same power settings. Different from conventional photocoagulation with CW laser, SP-Mode® enables the treatment of RPE cells with a slight temperature increase without destroying the RPE and photoreceptor cells. Laser is the best way to target RPE exogenously because laser light from 500nm to 800nm wavelength penetrates very well through the ocular media, and is also well absorbed in the melanin of the RPE cells, which has a high absorption coefficient. However, the longer the wavelength, the deeper the light penetrates and the temperature distribution may be shifted to the choroidal side [2]. The laser with 577nm yellow wavelength may be well absorbed by the RPE melanin; and thus, is suitable to treat RPE cells efficiently.
RPE, the cell monolayer lying in close proximity to the posterior side of the retina, is highly essential for the retinal functions, including light protection, vitamin-A cycle, phagocytosis (renewal) of photoreceptor outer segments, and transport of fluid, ion, metabolites and amino acids. These functions may directly and indirectly influence the function of photoreceptors and eventually of the whole retina. Therefore, the health of the RPE essentially affects the health of the entire retina. With SP-Mode®, it is possible to heat the RPE very mildly and activate the metabolism and functions of the RPE cells. Different preclinical studies have already suggested that this treatment could increase RPE’s pump function (water accumulation) [3] and secrete different proteins/ cytokines which can be beneficial for the visual functions [4-6]. In conclusion, the basic strategy of the treatment is warming RPE cells in order to lead to the “functional remodeling of the RPE and the subsequent stabilization of retinal homeostasis.”
CSC with TruScan 577 SP-Mode®
CASE 1
A 53-year-old man was referred to the university hospital with persistent CSC in his left eye for longer than 20 months. This was most likely triggered by a steroid (known as a risk factor) pulse treatment made for his hearing problem. Initially, he was prescribed nepafenac eye drops (NSAID), which showed no apparent therapeutic effect. On the first visit, his visual acuity in his right eye (OD) was (1.0) (20/20), but in his left eye (OS) it was (0.32) (20/63). In addition, he had highly hyperopic eyes (S+9.0), which is also one of the known risk factors of CSC. His intraocular pressure was normal in both eyes.
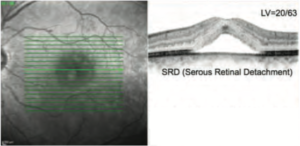
Figure 2 presents the result of the patient’s OCT at the first visit, demonstrating the subfoveal SRD. He started oral administration of eplerenone. Eplerenone is an aldosterone antagonist that is typically used for the treatment of chronic heart failure and high blood pressure, but has also been applied in CSC treatment as an off-label use. In this case, eplerenone showed almost no effect for 4 months, so he switched to acetazolamide (with potassium). Acetazolamide led to the reduction of the SRF at the beginning, but complete resolution was not achieved and the SRF was left persistently for another 6 months. He was then referred for retina laser therapy.
Treatment
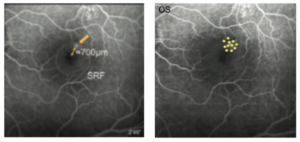
Figure 3 presents an FA image of his left eye, showing a clear single dye leakage point in the foveal to parafoveal region at supero-temporal to the fovea (Figure 3, left). The patient was treated with the TruScan 577 with SP-Mode® around the leakage point with the parameters set at a total duration of 200ms, spot size of 200μm, and duty cycle of 10%. The utilized laser contact lens was Area Centralis® (Volk, magnification 1.06x).
Power titration was conducted outside of the vascular arcade, starting with a power of 200mW and increased with 100mW increments until a mild white visible spot appeared (within several seconds after irradiation). In this case, the clear change was seen at 600mW. Then the power was reduced by 40mW to 560mW, at which the test spot was mildly visible. The power for CSC treatment is generally determined as 50% of the power of the threshold of visibility, so in this case, it was determined at 280mW (≈50% of 560mW). Here, eleven irradiations were applied around the leaking point (Figure 3, right). During treatment no spots were ophthalmoscopically visible and there were no other complications. The clinical course of this case is shown in Figure 4. As seen here, a significant SRF reduction was seen at 1.5 months after laser treatment and the SRF was completely resolved within 3-4 months. Neither scar formation nor structural change was detected at the irradiated points in any retinal imaging including OCT and autofluorescence.
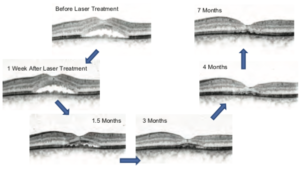
Figure 5 presents the changes of the central macular thickness (CMT) and visual acuity over time of this patient. This clearly shows that the laser treatment with SP-Mode® was effective in the resorption of the SRF as well as in the improvement of his visual acuity. Despite the persistent SRF under treatment of eplerenon and acetazolamide, the single laser treatment with SP-Mode® led to the rapid reduction of the SRF and a complete resorption within 3-4 months.
The patient commented that “the treatment itself was painless, quick and well tolerable. Now I am glad that I am free from systemic medicines.”

CASE 2
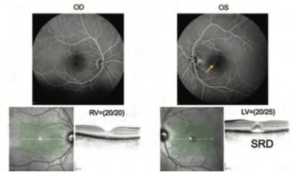
A 45-year-old female was diagnosed with her first episode of CSC after she had noticed a visual disturbance in her left eye for several months. The decreased visual acuity was first pointed out by her optician when she wanted to change her glasses. Her first ophthalmologist prescribed her acetazolamide, but there was no effect or improvement in the first three weeks, and she finally showed a drug intolerance to acetazolamide. Therefore, acetazolamide was ceased and she was referred to the university hospital. Her visual acuity at the first visit was OD = (1.0) (20/20), and OS = (0.8) (20/25), and her IOP showed normal in both eyes.
As shown in Figure 6, a typical subfoveal SRD was presented in her OCT. Her FA shows the mild and faint hyperfluorescence region. Upon closer look at her FA result, there were diffusely distributed weak hyperfluorescence areas in the foveal to parafoveal regions, suggesting weak leakage or staining due to mild RPE damage (Figure 6).
Treatment
Laser treatment with SP-Mode® was conducted evenly across the SRF area (except foveal center) as shown in Figure 7.
Test irradiation was conducted, outside of the vascular arcade, starting with a power of 200mW. The mild burn was seen at 600mW, and thus the treatment power was determined as 300mW according to the same strategy as above in case 1 (50% power of the threshold of visibility).
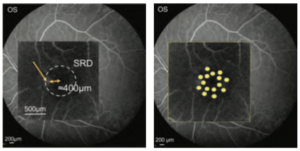
As shown below (Figure 7, left), the closest leakage point was about 400μm from the center of the fovea. In CSC cases with leakage points within the fovea (up to about 700μm), such as this case, the treatment power should be reduced by about 80-90% from the determined one, and no irradiation should be made within the central 500μm-area (in the author’s hospital, so far). For this patient, 8 irradiations were applied on the outer area with 300mW, and 7 further spots with the slightly reduced power at 260mW in the inner area (Figure 7, right). With this patient, no spots were ophthalmoscopically visible during treatment and no spots were detectable with any imaging modality.
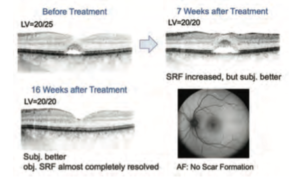
Seven weeks after treatment, subjective visual acuity was increased from 20/25 to 20/20 with less complaints by the patient; however, her OCT revealed increased SRF. It was decided to wait longer. In the next follow-up, 16 weeks after treatment (4 weeks delayed due to the outbreak of COVID-19), the SRF had been completely resolved (Figure 8). Subjective visual symptoms also further improved.
Conclusion
These cases show that SP-Mode® Microsecond Laser Technology enables treating the macula without risk of scar formation and scotoma. It is a safe and effective procedure to treat different types of CSC, with a localized leakage point or diffuse leakage, and with or without macular edema. No ocular or systemic side effects have been confirmed. It is important to perform test irradiations because every patient has a different extent of light absorption. The treatment power set at 40-50% of the visibility threshold seems to be safe and effective to treat CSC without scar formations, and it is clinically validated in previous reports [7,8]. As learned by the second case, it is important for the ophthalmologist and the patient to wait and see the apparent clinical effect. The time period of the SRF reduction varies among patients, thus it is recommended to wait for at least 3 months after treatment. Furthermore, this minimally-invasive method enables repeated laser treatments which can reduce patients’ physical, mental, and economical burdens of repeated injections or taking systemic drugs.